
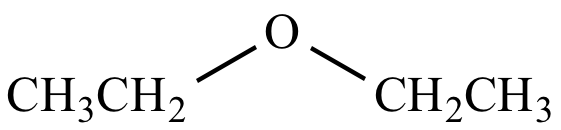
This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. The cookies is used to store the user consent for the cookies in the category "Necessary". The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". The cookie is used to store the user consent for the cookies in the category "Analytics". These cookies ensure basic functionalities and security features of the website, anonymously. Necessary cookies are absolutely essential for the website to function properly. A typical example is diethyl ether, commonly known as “ether” (ethoxyethane, CH 3-CH 2-O-CH 2-CH 3). Ethers are more polar than alkenes but not as polar as alcohols, esters, or amides of comparable structure.Įther is the general name for a class of organic chemical compounds characterized by molecules that contain an ether functional group-an oxygen atom directly bound to two hydrocarbon (alkyl or aryl) groups. The C–O–C bond angle in the functional group is about 110°, and the C–O dipoles do not cancel out. It is currently being used as a substitute for liquefied petroleum gas.Įthers are slightly polar. Also known as DME, it functions as a reagent in chemistry laboratories. The two lone pairs repel each other and thus force the bonds a little bit closer together.ĭimethyl ether is an organic compound that is used to make other molecules. The C-O-C bond angle in dimethyl ether is 108 Degrees because the oxygen forms two bonds and still has two lone pairs on the oxygen. What is the bond angle of dimethyl ether? You might reasonably expect it to be insolube in nonpolar solvents. Unlike alcohols, ethers are not acidic and usually do not react with bases.Īcetic acid, as a small, polar molecule capable of hydrogen bonding with water is very soluble in water. It is used extensively in the chemical industry and as an aerosol propellant. Under normal atmospheric conditions, DME is a colorless gas. What is dimethyl ether used for?ĭimethyl ether (DME) is a synthetically produced alternative to diesel for use in specially designed compression ignition diesel engines.

Cocaine, which is a nonpolar organic compound, dissolves readily in diethyl ether. This also makes diethyl ether perfect for the illicit production of pure cocaine, a powerful and highly addicting drug.
Therefore, diethyl ether is a great solvent for fats, waxes, oils, perfumes, alkaloids, and gums. Alkanes, alkenes, and alkynes are essentially non-polar and insoluble in water.ĭiethyl ether is a hard Lewis base that reacts with a variety of Lewis acids such as I2, phenol, and Al(CH3)3, and its base parameters in the ECW model are EB = 1.80 and CB = 1.63. (9) HYDROCARBON: There is very little intermolecular association because the carbon-hydrogen bond is non-polar. The boiling point, dipole moment, and dielectric constant of each solvent is included.Įthers as Solvents Because diethyl ether has a dipole moment, polar substances readily dissolve in it.ĭimethyl ether/Soluble in Are ethers polar or nonpolar?Įthers are essentially non-polar and insoluble in water. Table 1 presents a list of solvents that are commonly used in chemical reactions. Non-Polar Solvents Examples include benzene (C6H6), carbon tetrachloride (CCl4), and diethyl ether ( CH3CH2OCH2CH3). Is diethyl ether a polar or nonpolar solvent? 4 What is the bond angle of dimethyl ether?.1 Is diethyl ether a polar or nonpolar solvent?.


 0 kommentar(er)
0 kommentar(er)
